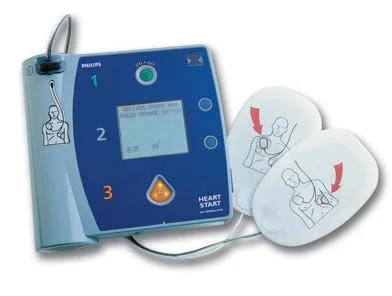
HeartStart FR2+ AED with ECG Display (Refurbished)
For professional and lay responder alike, HeartStart FR2+ was designed for fast, efficient operation.
SMART CPR - Patient specific treatment
The HeartStart SMART CPR evaluates key characteristics of the presenting ventricular fibrillation and determines the initial therapy: shock first, or CPR first quickly followed by a shock.
Easy hand-off - Allows a continuity of patient care
Advanced life support (ALS) trained professionals can easily switch the HeartStart FR2+ to manual mode, giving them more decision-making control. An ECG display is also available for critical review.
SMART Biphasic therapy - Safely delivers shock
Philips SMART Biphasic therapy uses a unique combination of high current - to increase effectiveness, and a low energy dose - to decrease side effects that are harmful to a fragile heart.
Built-in self test - Ready when needed
Your HeartStart FR2+ goes through a 120-point test before it leaves the factory. On the job, it conducts self tests daily, not just weekly. It performs over 80 different tests, including pads integrity with visual & attention-getting audible alerts.
Quick Shock feature - Reduce chest compressions
CPR is even more vital to survival than previously realized. Rapidly delivering a shock after chest compressions is critical. The HeartStart FR2+’s Quick Shock feature reduces the time between hands-off and shock delivery.
Simple to use - Clear, calm voice commands
Operation of the HeartStart FR2+ is guided by clear, streamlined, confident, and concise, voice commands: ideal for responders who are trained, drilled, and ready to save a life.
Includes:
1 FR2+ AED
1 Set of instructions for use
1 Standard battery
2 Pair defibrillator
1 Quick Reference Guide
Specifications:
Waveform:
Truncated exponential biphasic. Waveform parametersadjusted as a function of patient impedance
Therapy:
Adult defibrillation peak current: 32A (150J nominal) into a 50 ohm load; Pediatric defibrillation (utilizes infant/child pads): 19A (50J nominal) into a 50 ohm load
Charge Time from Shock Advised: Typically less than 10 seconds
Charge Time in Manual Mode: Typically less than 5 seconds
Shock-to-Shock Cycle Time: Typically less than 15 seconds (including analysis time) in automated mode
Protocol:
Text and voice prompts guide user through protocol. Follows pre-configured settings. Can be modified with the training and administration pack (PHIM3864A)
Shock Delivery: Via defibrillator pads placed in anterior-anterior (lead II) position for adult defibrillation and anterior-posterior for infant/child defibrillation
Controls: On/Off, Shock, screen contrast/option buttons
Indicators: LCD screen, beeper, audio speakers, status indicator. Shock button connector socket LED
Advanced Mode: Configurable protocol
ECG Display:
Screen: High-resolution LCD with bright back-light
Screen Dimensions: 2.8 inches wide x 2.3 inches high (7.0 cm x 5.8 cm)
Display Range: Differential: ±2 mV full scale (nominal)
Sweep Speed: 23 mm/second (nominal)
Frequency Response: 1 Hz to 20 Hz (-3dB) (nominal)
Sensitivity: 1.16 cm/mV (nominal)
Heart Rate: 30 to 300 beats per minute updated each analysis period during monitoring
Monitored Lead: Anterior-anterior (lead II) placement with adult defibrillation pads or ECG Assessment Module (PHIM3860A) only
Patient Analysis System:
Patient Analysis: Per protocol, evaluates patient ECG and signal quality to determine if rhythm is shockable, and evaluates connection impedance for proper defibrillation pad contact
Sensitivity/Specificity: Meets AAMI DF-80 guidelines and AHA recommendationsfor adult defibrillation (Circulation 1997;95:1677-1682)
SMART CPR (configurable):
Enables support of an automated or user-initiated CPR interval prior to defibrillation shockable rhythm. Once the decision is made, FR2+ provides the responder with the appropriate prompts.
SMART AUTO 1: advises CPR for patients with a presenting rhythm typical of very long-duration cardiac arrest.
SMART AUTO 2: advises CPR for an expanded group of patients inclusive of those in Auto 1, having a rhythm typical of long duration cardiac arrest
USER: user-initiated CPR Pause interval. Supports a protocol under which the responder decides whether to perform CPR first. A Pause-for-CPR button is enabled, and can be pressed at the responder’s discretion.
The AUTO 1 and AUTO 2 settings automate the decision of whether to provide CPR first or deliver a shock first based on the amplitude and frequency of the presenting
Quick Shock Able to deliver a shock in typically less than 10 seconds after the end of a CPR interval.
Infant/Child Defibrillation Pads (PHIM3870A):
Patient: Under 8 years or 55 lbs / 25 kg
Defibrillator Compatibility FR2-series: (FR2 and FR2+) automated external defibrillator only
Configuration: 1 set per package
How Supplied: Disposable self-adhesive pads with cable and connector
Therapy Delivered: Reduces defibrillator shock peak current to 19A (50 Joules nominal) into a 50 ohm load
Sureface Area: Meets AAMI DF-80 guidelines
Cable Length: Approximately 48 lbs / 122 cm
Medical Control/Recording Features:
Standard Event Review: Elapsed time and number of shocks are displayed on screen
Enhanced Event Review: Optional data card (PHIM3854A) expands the above on-screen event review capabilities. Review chronological events in detail including ECG 8 hours of event and ECG recording or one hour if voice recording is activated
FR2 Series Standard Battery (PHIM3863A):
Type: 12 VDC 4.2 Ah lithium manganese. Disposable, recyclable, long-life, primary cells
Capacity: Minimum 300 shocks or 12 hours of operating time (EN 60601-2-4:2003)
Install-by Date Battery is labeled with an install-by date at least 5 years from date of manufacture
Standby Life: Defines how long the battery will power the AED in standby operation within the standby temperature range (one battery insert test and no uses). 4 years minimum when battery is installed by the install-by date (5 years typical)
Environmental/Physical Requirements:
Sealing: Meets IEC529 class IP54 with battery and data card tray installed
Temperature : Operating: 32º - 122º F / 0º - 50º C; Standby: 32º - 109º F / 0º - 43º C - Standby applies to AED with battery installed and stored with defibrillation pads
Humidity: Operating: 0% to 95% relative humidity (non-condensing); Standby: 0% to 75% relative humidity (non-condensing)
Altitude: -500 to 15,000 feet per MIL-STD-810E 500.3 Procedure II
Aircraft: Method: RTCA/DO-160D: 1997 Section 21 (Category M - Charging)
Shock/Drop Abuse Tolerance: 1 meter any edge, corner or surface. MIL-STD-810E 516.4 Procedure IV
Vibration: MIL-STD-810E 514.4-17
EMI: Requirements: Tested to EN60601-1-2 Limits; Radiated: Method EN55011: 1998 Group 1 Level B; Immunity: Method EN61000-4-3:1998 Level 2
Automated and User-activated Self-tests:
Automatic Self-tests: Tests internal circuitry, waveform delivery system, and battery capacity. Verifies calibration of key circuits monthly
Automatic Self-test Frequency: Daily when stored within operating environmental conditions
Status Indication: Dynamic visual and audible indication of self-test results. Indicates device readiness
Battery Insertion Test: Upon battery insertion, extensive automatic self-tests and user interactive tests check device readiness. Verifies calibration of key circuits
Automatic Standby Temperature Monitoring: Instrument automatically monitors temperature and warns user if device is stored outside of standby temperature range
Dimensions:
Height: 8.6" / 21.8 cm.
Width: 8.6" / 21.8 cm.
Depth: 2.6" / 6.6 cm.
Weight:
With battery: 4.7 lbs / 2.1 kg.
Without battery: 3.9 lbs / 1.8 kg
For professional and lay responder alike, HeartStart FR2+ was designed for fast, efficient operation.
SMART CPR - Patient specific treatment
The HeartStart SMART CPR evaluates key characteristics of the presenting ventricular fibrillation and determines the initial therapy: shock first, or CPR first quickly followed by a shock.
Easy hand-off - Allows a continuity of patient care
Advanced life support (ALS) trained professionals can easily switch the HeartStart FR2+ to manual mode, giving them more decision-making control. An ECG display is also available for critical review.
SMART Biphasic therapy - Safely delivers shock
Philips SMART Biphasic therapy uses a unique combination of high current - to increase effectiveness, and a low energy dose - to decrease side effects that are harmful to a fragile heart.
Built-in self test - Ready when needed
Your HeartStart FR2+ goes through a 120-point test before it leaves the factory. On the job, it conducts self tests daily, not just weekly. It performs over 80 different tests, including pads integrity with visual & attention-getting audible alerts.
Quick Shock feature - Reduce chest compressions
CPR is even more vital to survival than previously realized. Rapidly delivering a shock after chest compressions is critical. The HeartStart FR2+’s Quick Shock feature reduces the time between hands-off and shock delivery.
Simple to use - Clear, calm voice commands
Operation of the HeartStart FR2+ is guided by clear, streamlined, confident, and concise, voice commands: ideal for responders who are trained, drilled, and ready to save a life.
Includes:
1 FR2+ AED
1 Set of instructions for use
1 Standard battery
2 Pair defibrillator
1 Quick Reference Guide
Specifications:
Waveform:
Truncated exponential biphasic. Waveform parametersadjusted as a function of patient impedance
Therapy:
Adult defibrillation peak current: 32A (150J nominal) into a 50 ohm load; Pediatric defibrillation (utilizes infant/child pads): 19A (50J nominal) into a 50 ohm load
Charge Time from Shock Advised: Typically less than 10 seconds
Charge Time in Manual Mode: Typically less than 5 seconds
Shock-to-Shock Cycle Time: Typically less than 15 seconds (including analysis time) in automated mode
Protocol:
Text and voice prompts guide user through protocol. Follows pre-configured settings. Can be modified with the training and administration pack (PHIM3864A)
Shock Delivery: Via defibrillator pads placed in anterior-anterior (lead II) position for adult defibrillation and anterior-posterior for infant/child defibrillation
Controls: On/Off, Shock, screen contrast/option buttons
Indicators: LCD screen, beeper, audio speakers, status indicator. Shock button connector socket LED
Advanced Mode: Configurable protocol
ECG Display:
Screen: High-resolution LCD with bright back-light
Screen Dimensions: 2.8 inches wide x 2.3 inches high (7.0 cm x 5.8 cm)
Display Range: Differential: ±2 mV full scale (nominal)
Sweep Speed: 23 mm/second (nominal)
Frequency Response: 1 Hz to 20 Hz (-3dB) (nominal)
Sensitivity: 1.16 cm/mV (nominal)
Heart Rate: 30 to 300 beats per minute updated each analysis period during monitoring
Monitored Lead: Anterior-anterior (lead II) placement with adult defibrillation pads or ECG Assessment Module (PHIM3860A) only
Patient Analysis System:
Patient Analysis: Per protocol, evaluates patient ECG and signal quality to determine if rhythm is shockable, and evaluates connection impedance for proper defibrillation pad contact
Sensitivity/Specificity: Meets AAMI DF-80 guidelines and AHA recommendationsfor adult defibrillation (Circulation 1997;95:1677-1682)
SMART CPR (configurable):
Enables support of an automated or user-initiated CPR interval prior to defibrillation shockable rhythm. Once the decision is made, FR2+ provides the responder with the appropriate prompts.
SMART AUTO 1: advises CPR for patients with a presenting rhythm typical of very long-duration cardiac arrest.
SMART AUTO 2: advises CPR for an expanded group of patients inclusive of those in Auto 1, having a rhythm typical of long duration cardiac arrest
USER: user-initiated CPR Pause interval. Supports a protocol under which the responder decides whether to perform CPR first. A Pause-for-CPR button is enabled, and can be pressed at the responder’s discretion.
The AUTO 1 and AUTO 2 settings automate the decision of whether to provide CPR first or deliver a shock first based on the amplitude and frequency of the presenting
Quick Shock Able to deliver a shock in typically less than 10 seconds after the end of a CPR interval.
Infant/Child Defibrillation Pads (PHIM3870A):
Patient: Under 8 years or 55 lbs / 25 kg
Defibrillator Compatibility FR2-series: (FR2 and FR2+) automated external defibrillator only
Configuration: 1 set per package
How Supplied: Disposable self-adhesive pads with cable and connector
Therapy Delivered: Reduces defibrillator shock peak current to 19A (50 Joules nominal) into a 50 ohm load
Sureface Area: Meets AAMI DF-80 guidelines
Cable Length: Approximately 48 lbs / 122 cm
Medical Control/Recording Features:
Standard Event Review: Elapsed time and number of shocks are displayed on screen
Enhanced Event Review: Optional data card (PHIM3854A) expands the above on-screen event review capabilities. Review chronological events in detail including ECG 8 hours of event and ECG recording or one hour if voice recording is activated
FR2 Series Standard Battery (PHIM3863A):
Type: 12 VDC 4.2 Ah lithium manganese. Disposable, recyclable, long-life, primary cells
Capacity: Minimum 300 shocks or 12 hours of operating time (EN 60601-2-4:2003)
Install-by Date Battery is labeled with an install-by date at least 5 years from date of manufacture
Standby Life: Defines how long the battery will power the AED in standby operation within the standby temperature range (one battery insert test and no uses). 4 years minimum when battery is installed by the install-by date (5 years typical)
Environmental/Physical Requirements:
Sealing: Meets IEC529 class IP54 with battery and data card tray installed
Temperature : Operating: 32º - 122º F / 0º - 50º C; Standby: 32º - 109º F / 0º - 43º C - Standby applies to AED with battery installed and stored with defibrillation pads
Humidity: Operating: 0% to 95% relative humidity (non-condensing); Standby: 0% to 75% relative humidity (non-condensing)
Altitude: -500 to 15,000 feet per MIL-STD-810E 500.3 Procedure II
Aircraft: Method: RTCA/DO-160D: 1997 Section 21 (Category M - Charging)
Shock/Drop Abuse Tolerance: 1 meter any edge, corner or surface. MIL-STD-810E 516.4 Procedure IV
Vibration: MIL-STD-810E 514.4-17
EMI: Requirements: Tested to EN60601-1-2 Limits; Radiated: Method EN55011: 1998 Group 1 Level B; Immunity: Method EN61000-4-3:1998 Level 2
Automated and User-activated Self-tests:
Automatic Self-tests: Tests internal circuitry, waveform delivery system, and battery capacity. Verifies calibration of key circuits monthly
Automatic Self-test Frequency: Daily when stored within operating environmental conditions
Status Indication: Dynamic visual and audible indication of self-test results. Indicates device readiness
Battery Insertion Test: Upon battery insertion, extensive automatic self-tests and user interactive tests check device readiness. Verifies calibration of key circuits
Automatic Standby Temperature Monitoring: Instrument automatically monitors temperature and warns user if device is stored outside of standby temperature range
Dimensions:
Height: 8.6" / 21.8 cm.
Width: 8.6" / 21.8 cm.
Depth: 2.6" / 6.6 cm.
Weight:
With battery: 4.7 lbs / 2.1 kg.
Without battery: 3.9 lbs / 1.8 kg
For professional and lay responder alike, HeartStart FR2+ was designed for fast, efficient operation.
SMART CPR - Patient specific treatment
The HeartStart SMART CPR evaluates key characteristics of the presenting ventricular fibrillation and determines the initial therapy: shock first, or CPR first quickly followed by a shock.
Easy hand-off - Allows a continuity of patient care
Advanced life support (ALS) trained professionals can easily switch the HeartStart FR2+ to manual mode, giving them more decision-making control. An ECG display is also available for critical review.
SMART Biphasic therapy - Safely delivers shock
Philips SMART Biphasic therapy uses a unique combination of high current - to increase effectiveness, and a low energy dose - to decrease side effects that are harmful to a fragile heart.
Built-in self test - Ready when needed
Your HeartStart FR2+ goes through a 120-point test before it leaves the factory. On the job, it conducts self tests daily, not just weekly. It performs over 80 different tests, including pads integrity with visual & attention-getting audible alerts.
Quick Shock feature - Reduce chest compressions
CPR is even more vital to survival than previously realized. Rapidly delivering a shock after chest compressions is critical. The HeartStart FR2+’s Quick Shock feature reduces the time between hands-off and shock delivery.
Simple to use - Clear, calm voice commands
Operation of the HeartStart FR2+ is guided by clear, streamlined, confident, and concise, voice commands: ideal for responders who are trained, drilled, and ready to save a life.
Includes:
1 FR2+ AED
1 Set of instructions for use
1 Standard battery
2 Pair defibrillator
1 Quick Reference Guide
Specifications:
Waveform:
Truncated exponential biphasic. Waveform parametersadjusted as a function of patient impedance
Therapy:
Adult defibrillation peak current: 32A (150J nominal) into a 50 ohm load; Pediatric defibrillation (utilizes infant/child pads): 19A (50J nominal) into a 50 ohm load
Charge Time from Shock Advised: Typically less than 10 seconds
Charge Time in Manual Mode: Typically less than 5 seconds
Shock-to-Shock Cycle Time: Typically less than 15 seconds (including analysis time) in automated mode
Protocol:
Text and voice prompts guide user through protocol. Follows pre-configured settings. Can be modified with the training and administration pack (PHIM3864A)
Shock Delivery: Via defibrillator pads placed in anterior-anterior (lead II) position for adult defibrillation and anterior-posterior for infant/child defibrillation
Controls: On/Off, Shock, screen contrast/option buttons
Indicators: LCD screen, beeper, audio speakers, status indicator. Shock button connector socket LED
Advanced Mode: Configurable protocol
ECG Display:
Screen: High-resolution LCD with bright back-light
Screen Dimensions: 2.8 inches wide x 2.3 inches high (7.0 cm x 5.8 cm)
Display Range: Differential: ±2 mV full scale (nominal)
Sweep Speed: 23 mm/second (nominal)
Frequency Response: 1 Hz to 20 Hz (-3dB) (nominal)
Sensitivity: 1.16 cm/mV (nominal)
Heart Rate: 30 to 300 beats per minute updated each analysis period during monitoring
Monitored Lead: Anterior-anterior (lead II) placement with adult defibrillation pads or ECG Assessment Module (PHIM3860A) only
Patient Analysis System:
Patient Analysis: Per protocol, evaluates patient ECG and signal quality to determine if rhythm is shockable, and evaluates connection impedance for proper defibrillation pad contact
Sensitivity/Specificity: Meets AAMI DF-80 guidelines and AHA recommendationsfor adult defibrillation (Circulation 1997;95:1677-1682)
SMART CPR (configurable):
Enables support of an automated or user-initiated CPR interval prior to defibrillation shockable rhythm. Once the decision is made, FR2+ provides the responder with the appropriate prompts.
SMART AUTO 1: advises CPR for patients with a presenting rhythm typical of very long-duration cardiac arrest.
SMART AUTO 2: advises CPR for an expanded group of patients inclusive of those in Auto 1, having a rhythm typical of long duration cardiac arrest
USER: user-initiated CPR Pause interval. Supports a protocol under which the responder decides whether to perform CPR first. A Pause-for-CPR button is enabled, and can be pressed at the responder’s discretion.
The AUTO 1 and AUTO 2 settings automate the decision of whether to provide CPR first or deliver a shock first based on the amplitude and frequency of the presenting
Quick Shock Able to deliver a shock in typically less than 10 seconds after the end of a CPR interval.
Infant/Child Defibrillation Pads (PHIM3870A):
Patient: Under 8 years or 55 lbs / 25 kg
Defibrillator Compatibility FR2-series: (FR2 and FR2+) automated external defibrillator only
Configuration: 1 set per package
How Supplied: Disposable self-adhesive pads with cable and connector
Therapy Delivered: Reduces defibrillator shock peak current to 19A (50 Joules nominal) into a 50 ohm load
Sureface Area: Meets AAMI DF-80 guidelines
Cable Length: Approximately 48 lbs / 122 cm
Medical Control/Recording Features:
Standard Event Review: Elapsed time and number of shocks are displayed on screen
Enhanced Event Review: Optional data card (PHIM3854A) expands the above on-screen event review capabilities. Review chronological events in detail including ECG 8 hours of event and ECG recording or one hour if voice recording is activated
FR2 Series Standard Battery (PHIM3863A):
Type: 12 VDC 4.2 Ah lithium manganese. Disposable, recyclable, long-life, primary cells
Capacity: Minimum 300 shocks or 12 hours of operating time (EN 60601-2-4:2003)
Install-by Date Battery is labeled with an install-by date at least 5 years from date of manufacture
Standby Life: Defines how long the battery will power the AED in standby operation within the standby temperature range (one battery insert test and no uses). 4 years minimum when battery is installed by the install-by date (5 years typical)
Environmental/Physical Requirements:
Sealing: Meets IEC529 class IP54 with battery and data card tray installed
Temperature : Operating: 32º - 122º F / 0º - 50º C; Standby: 32º - 109º F / 0º - 43º C - Standby applies to AED with battery installed and stored with defibrillation pads
Humidity: Operating: 0% to 95% relative humidity (non-condensing); Standby: 0% to 75% relative humidity (non-condensing)
Altitude: -500 to 15,000 feet per MIL-STD-810E 500.3 Procedure II
Aircraft: Method: RTCA/DO-160D: 1997 Section 21 (Category M - Charging)
Shock/Drop Abuse Tolerance: 1 meter any edge, corner or surface. MIL-STD-810E 516.4 Procedure IV
Vibration: MIL-STD-810E 514.4-17
EMI: Requirements: Tested to EN60601-1-2 Limits; Radiated: Method EN55011: 1998 Group 1 Level B; Immunity: Method EN61000-4-3:1998 Level 2
Automated and User-activated Self-tests:
Automatic Self-tests: Tests internal circuitry, waveform delivery system, and battery capacity. Verifies calibration of key circuits monthly
Automatic Self-test Frequency: Daily when stored within operating environmental conditions
Status Indication: Dynamic visual and audible indication of self-test results. Indicates device readiness
Battery Insertion Test: Upon battery insertion, extensive automatic self-tests and user interactive tests check device readiness. Verifies calibration of key circuits
Automatic Standby Temperature Monitoring: Instrument automatically monitors temperature and warns user if device is stored outside of standby temperature range
Dimensions:
Height: 8.6" / 21.8 cm.
Width: 8.6" / 21.8 cm.
Depth: 2.6" / 6.6 cm.
Weight:
With battery: 4.7 lbs / 2.1 kg.
Without battery: 3.9 lbs / 1.8 kg